Welcome to your Chemistry final
114. The number of atomic orbitals in the valence shell Ca atom are:
115. d-elements in the periodic table are:
116. How many are the acceptor atoms involved in formation of hydrogen bonds between guanine and cytosine:
117. Is it possible to find the enthalpy of CO(g) from the reaction, if we know:
\[CO_2(g)+H_2(g) -> CO(g)+H_2O(g)+ΔH_f\]
118. The two molecules represented below are:
119. Which of these statements describes a condensation reaction?
121. The alcohol that contains two alkyl groups attached to the carbon bonded to the –OH group is a
122. Which type of organic compound does not contain a carbonyl group?
123. Select the monosaccharide (s).
124. In the IUPAC nomenclature system, the name of which of the following would end in -al?
125. The general formula for an ether is
126. Which of the following is classified as an aldehyde?
127. What is the correct classification for this alcohol?
128. \[CH_3-CH_2-C(=O)NH_2\] is called a(n)___________
129. During an endothermic reaction the compounds with less energy are:
130. The heat of formation of pure carbon in different allotronic forms:
131. Which compound can form hydrogen chemical bonds:
134. The principal difference between fructose ans glucose is that__________
135. Which one of the following statement is true?
136. I, 2-dichlorethene has
138. Which of the following is classified as an aldehyde?
139. The general formula fo an ether is
140. _________________ is a disaccharide
141. cis-trans isomerism is
142. Which of the statements are true?
Methane, ethane,hexane are?
I. homologies
II. are alkanes
III. are isomers
IV. are alkenes
143. Which of the properties of simple aromatic coumpound is true?
144. The name of the compound
\[CH_3CH_2C(CH)_2CH_2CH_3\] is:
145. If the concentration of a reactant is increase at constant temperature, then the equilibrium constant:
146. At equilibrium, concentration is of reactants:
147. The rate of a chemical reaction decreases if:
148. When temperature is increased the rate increases:
149. During an endothermic reaction the compounds with less energy are:
150. The heat of formation of pure carbon in diffwrent allotropic forms:
151. Which one correctly depicts a primary amine?
152. Which choice gives the structures of the reaction products when the ester below is hydrolyzed in acidic solution?
153. Which type of organic compound does not contain a carbonyl group?
154. Select the monosaccharide (s).
155. What is the IUPAC name of the compound shown?
156. Which of the compound reacts most readily with aqueous ammonia?
157. Which one of the following bases is strong: Ca(OH)2, Al(OH)3, NH4OH, C5H5N
158. Which salt when dissolved in water would form a basic solution: KClO4, KCl, CH3COOK, KNO3
159. In the reaction below, what atoms or ions are being oxidized and reduced, respectively.
\[HNO_2 +\,→\,NO + I3 -\]
160. Which of the statements are true?
I Metals have high melting and boiling points
II Metals have low boiling point
III Metals are good conductors of heat and electricity
IV Metal do not react with oxygen
161. Which of the statements are true?
I. oxides of metals react with water to form hydroxides
II. oxides of metals react with water to form acids
III. oxides of non-metals react with water to form acids
IV. oxides of non-metals do not react with water
- II and IV
- I and III
- I and IV
- II and IV
162. The compounds CH3COCH2CH2CH3 and CH3CH2CH2CH2CH2OH are:
164. In which compound there is ionic chemical bond:
165. The shared electron pair in a covalent non-polar bound spatial is localized:
166. The thermal effect of chemical reaction represents:
167. During an exothermic reaction the compounds with less energy are:
168. If the activation energy is decreased, the reaction rate:
169. The rate of a first order reaction is:
170. Equilibrium can be established during:
171. the equilibrium constant of endothermic reaction depends on:
172. The compound below is a(n) __________.
173. The hybridization of the central carbon atom in an aldehyde is _________.
174. The secondary structure of a protein is the result of _______ bonding.
175. The oxidation of ethanol produces _______.
176. The following reaction would produce a(n) _______.
177. Proteins are biopolymers formed via multiple condensation coupling of which two functional groups?
178. Which one of the following compounds is an isomer of :
\[CH_3CH_2CH_2CH_2OH\]
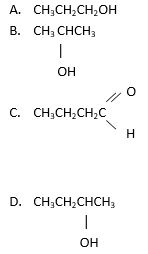
179. Which structure below represents a ketone?
180. Which one of the following is alkyne?
181. Which of the following is alkane:
182. What is the hybridization of each C atom in \[C_2H_4?\]
183. For which of the compounds below are cis-trans isomers possible:
184. What of the non-metals is usually used to kill microorganisms in water?
185. The number of protons in the nucleus of a chemical element determines:
186. The total sum of protons and neutrons in the nucleus of a chemical element determines:
187. Where you can find double bond in the following six compounds:
1. NH3Cl; (2) HMnO4; (3) C2H2; (4) HClO4; (5) K2CrO4; (6) HClO;
188. What are the possible oxidation states of sulphur:
189. During an endothermic reaction:
190. In which row the heat of formation of the gases constantly increases:
191. The chemical reaction rate is fastest when occurs between:
192. When temperature is decreased the rate of a chemical reaction:
193. The equilibrium constant:
194. The equilibrium constant:
195. The electrons in the first electron shell have:
196. The maximal number of electrons in the second electron shell is:
197. The nucleus of an atom:
198. which bands are formed by a carbon atom with sp2 hybridization?
199. What is the symbol for an ion which has 8 protons and 10 electrons?
200. Select the formula of potassium dichromate:
201. What are the products of reaction Zn + CuSO4→ :
202. In a redox reaction, there must be:
203. What is the name of the compound below:
204. What is the IUPAC name of the compound shown:
205. Which one of the following ions has the electron configuration
206. The net ionic equation for the hydrolysis of the salt
207. Which of the following best describes a solution of acid?
Litmus Colour Reaction with Zn
208. Which of the following is the product of the reaction of 1-hexyne with 1 mol of
209. Which compound undergoes the substitution reaction with NaCN?
210. Which of the following alcohols gives the best yield of diakyl either by heating with a trace of sulfuric acid?
211. The correct IUPAC name for the following compound is:
212. Which reactionis NOT possible with acetic acid?
213. Peptide bonds can be classified as:
214. Which one correctly depicts a quaternary ammonium ion?
215. Which of the following compound is NOT a monosaccharide?
216. Consider the following statement concerning the effect of the methyl group, - CH3, on an electrophil aromatic substitution:
I. The methyl group will activate the aromatic ring.
II. The methyl group will deactivate the aromatic ring.
III The methyl group will be meta-director.
IV. The methyl group will be an ortho- and a para-director.
217. Which statement about the carbonyl group os alderhydes is true?
I. The carbon atom in the carbonyl group can attract nucleophiles.
II. The carbon atom in the carbonyl group can attract electrophiles.
III. The carbonyl group undergoes addition reaction.
IV. The carbon atom in the carbonyl group can attract Lewis acids
218. Which of the following is the product of the reaction of butanal with H2/Pt:
219. Soaps are:
I. lond-chain esters
II. Long-chain fatty acids
III. Sodium salts of long-chain carboxylic acids
IV. Both polar and nonpolar molecules
220. Of the two pairs of acids, which one is stronger in each pair?
221. Sugars are examples of what type of molecule?
222. What is the major functional group in the following compound?
224. The reaction of an alcohol and a carboxylic acid yields
225. The name for the compound with the formula
\[CH_3CH_2CH_2CH_2OH\] is
226. Markovnikov's rule is a reaction of
227. How many isomers are possible for dibromobenzene:
228. The IUPAC name of para-dibromobenzene is:
229. What is the IUPAC name of
\[CH_3CH_2CH_2CH(CH_3)CH_3\]?
230. What is the next step in balancing the half reaction:
\[Cr_2O_7{^{2-}} → 2Cr^{3+}\] ?
231. What is the next step in balancing the half reaction:
\[SO_3^{{2-}}+H_2O→SO_4^{{2-}}+2H^+\] ?
232. The oxidation number of C in
234. \[Ag^+_{(ag)}+I^-_{(ag)} → AgI_{(s)}\] is example of:
235. The isotopes of a chemical element have:
236. If chnges in the nucleus of a chemical element occurs, the observed phenomena are:
238. How many single electrons have a phosphorus atom in not exited state:
239. Electron configuration of phosphorus is:
240.The chemical reaction rate is fastest when occurs between:
241. If the temperature of a chemical reaction is increased, then the equlibrium constant:
242. \[CH_3-CH_2-C(=O)NH_2\] is called a(n)_______________________
243. The principle difference between fructose and glucose is that_______.
244. 1, 2-dichlorethene has
245. Which of the following compounds does not contain a - C=O bond?
246. __________________is a monosaccharide
247. Which of the statments are true?
Methane, Ethane, Hexane are:
I. homologies
II. are alcanes
III. are isomers
IV. are alkenes